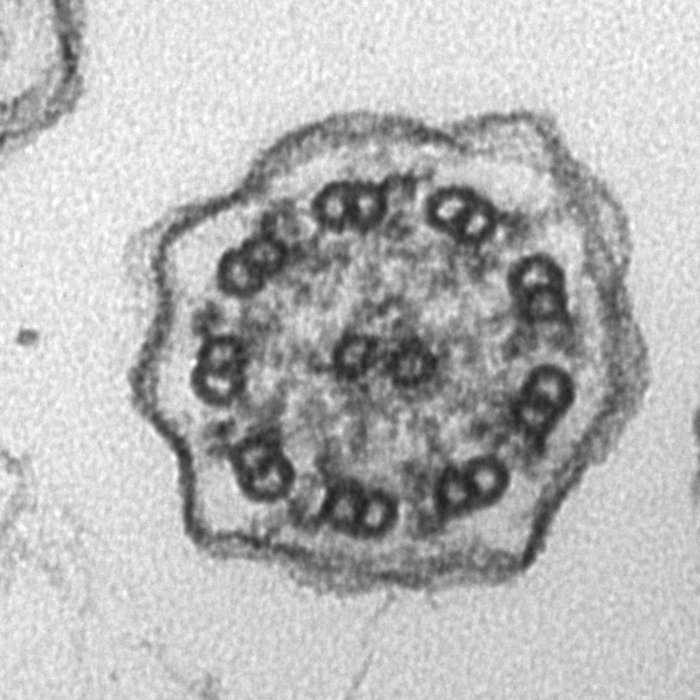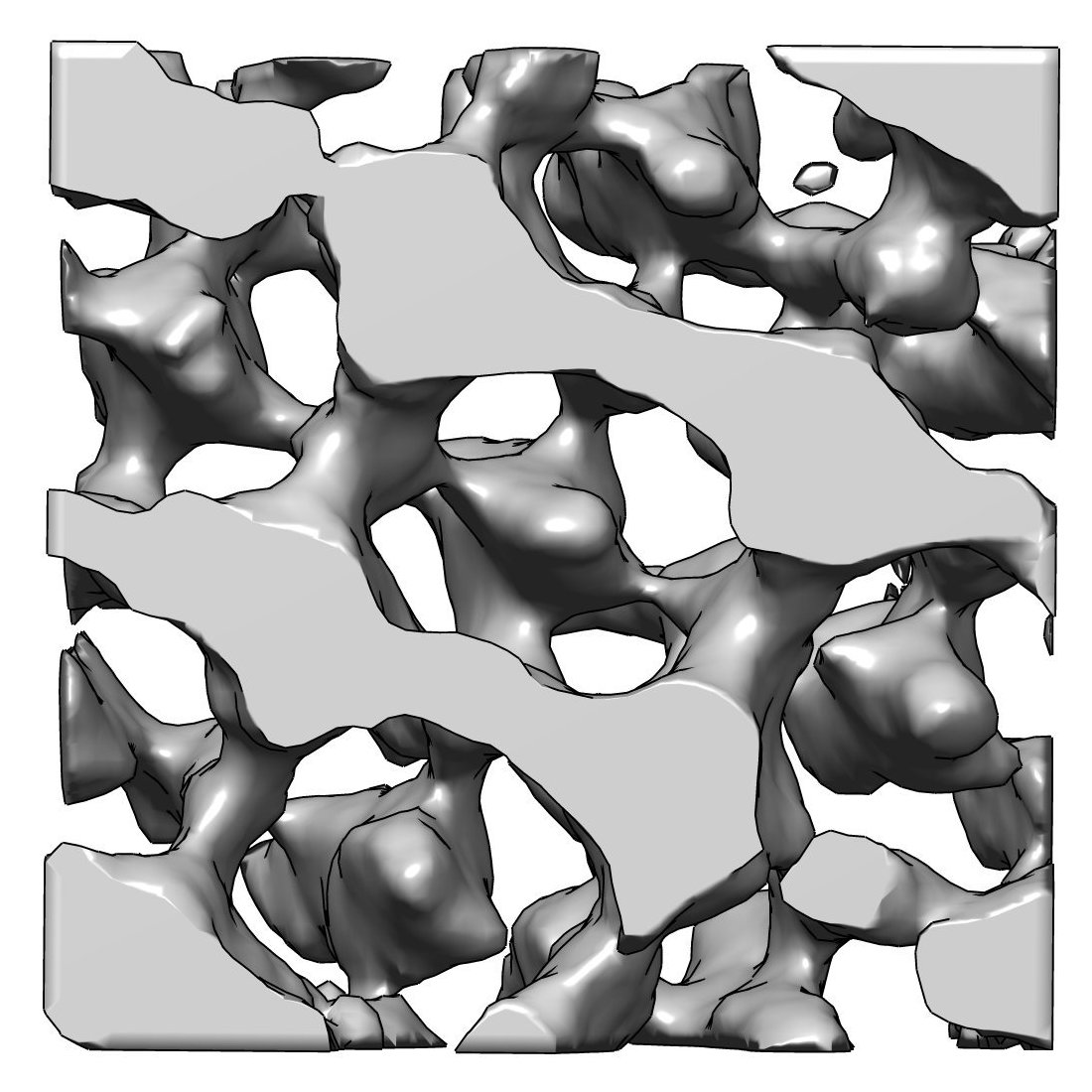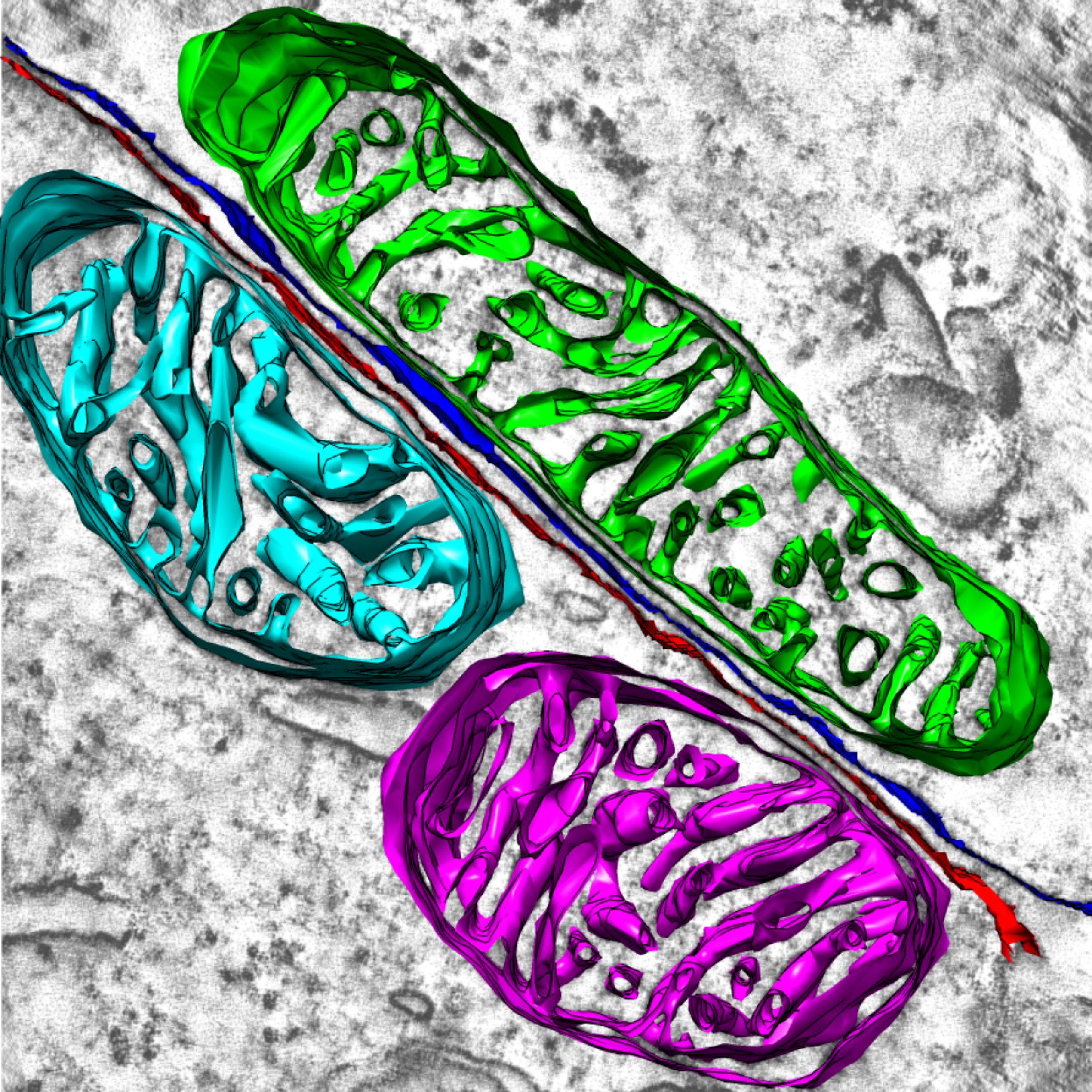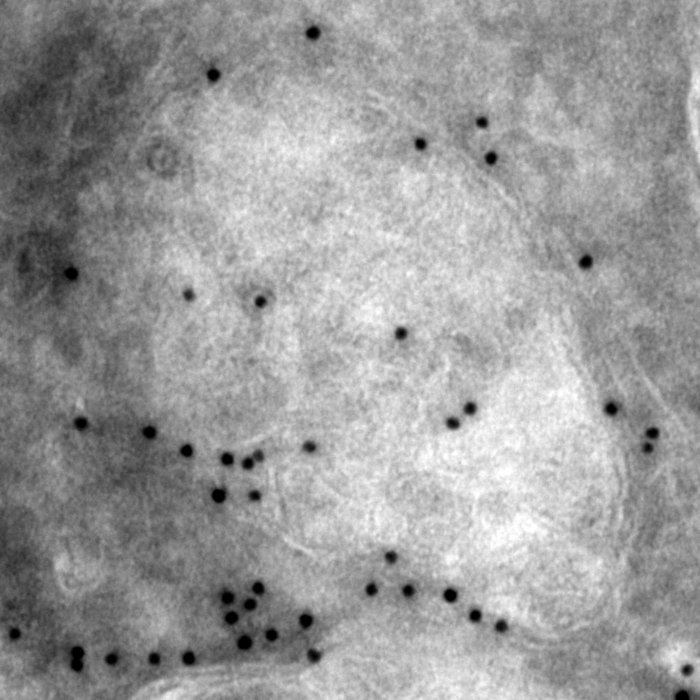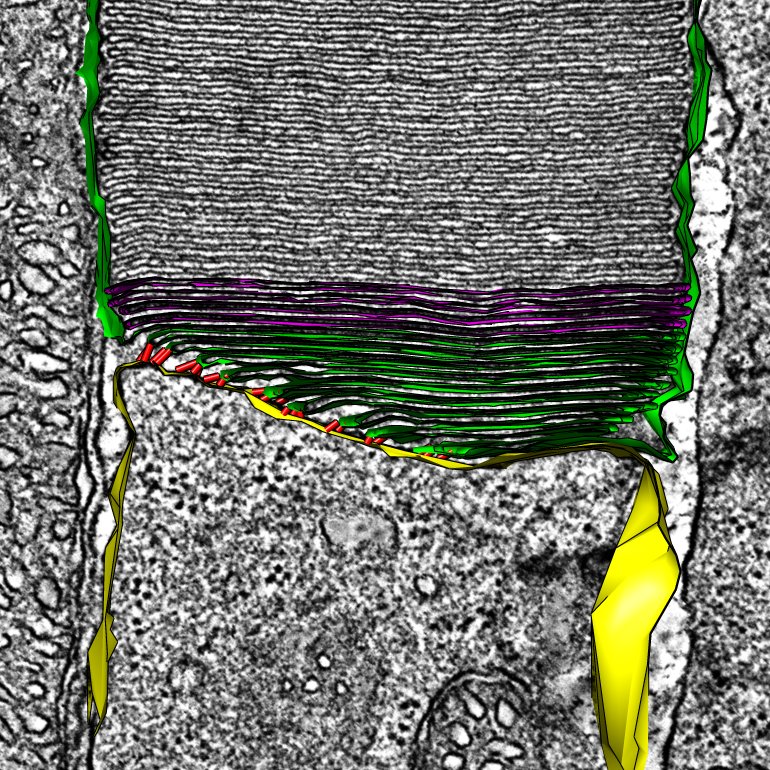
Rod Photoreceptor Disc Renewal
A slice from an electron tomogram that includes an overlay of a model showing the structure of the discs in the light detecting outer segment region of a rod photoreceptor. These disc structures are replaced on a daily basis from evaginations of the plasma membrane (shown in green).